What is Sulphate content testing?
Sulphate content testing in concrete quantifies soluble sulphates to assess the risk of sulphate attack, crucial for ensuring concrete durability and structural integrity.
How does Sulphate content testing work?
Sulphate content testing in concrete works by acid digestion of a powdered concrete sample, followed by sulphate determination in the solution through gravimetry.
Sulphate ions, dissolved by decomposing cement with hydrochloric acid, are precipitated at a pH between 1 and 1.5 by a solution of barium chloride (SO42– + Ba2+ = BaSO4).
The precipitation is carried out at boiling point with the weight of the precipitate measured to calculate the sulphate content.
This test assesses the risk of both internal sulphate attack and external sulphate attack creating expansive ettringite, thaumasite or gypsum forming reactions that crack the concrete from within.
What is Sulphate content testing used for?
Deterioration process | Defects | Control of repairs |
- | - |
How do I carry out Sulphate content testing?
Based on the procedures outlined in BS EN 196-2:2013 a simple step by step guide to carry out sulphate content testing includes the following steps:
Obtain cylindrical cores (75-150 mm diameter) from various, differently exposed or deteriorated members of the structure.
Reduce at least 1kg of concrete to a fine powder with particle size less than 105 µm.
Select a 1 (±0.05) g sub sample and place in a 250ml beaker with 90g of cold water.
While stirring vigorously, preferably with a magnetic stirring device add 10 ml of hydrochloric acid.
Heat the solution gently and crush the sample with a glass stirring rod.
Stir until the decomposition of the cement is complete.
Allow the solution to digest for 15 mins at a temperature just below boiling point.
Place filter paper over the top of a 400ml beaker and pass the residue through it.
Wash the precipitate five to six times and rinse the base of the filter stem with a few drops of water.
Wash the filter paper and its contents with several millilitres of water and collect in a test tube.
Add some silver nitrate solution to determine if all chlorides have been removed. If not continue washing while carrying out periodic checks until the silver nitrate test is negative.
Adjust the total solution volume to about 250ml by adding water; afterwards, if necessary, adjust the pH of the solution to between 1 and 1.5 using hydrochloric acid.
Bring solution to the boil and boil for 5 mins.
The substance should be fully clear at this point; if not start the test again using a new sub sample.
While stirring vigorously with the solution maintained at boiling point, add drop by drop 10ml of near boiling barium chloride solution.
Boil for 15 minutes to allow the precipitate to form from the barium and sulphates.
Allow the solution to stand for 12 to 24 hours below boiling temperature but above 60 °C, taking care to avoid concentration by evaporation.
Filter the precipitate through fine filter paper and wash thoroughly with boiling water until free from Cl– ions, tested by the silver nitrate test.
Ignite at (925 ± 25) °C in 15-minute intervals followed each time by cooling and weighing. This is repeated until the mass difference is less than 0.0005g with the final measured mass the constant mass of the precipitate.
Calculate sulphate content expressed as SO3 based on the original mass of the sample and the mass of the BaSO4 precipitate using Equation 1.
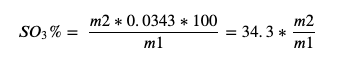
Equation 1. Sulphate percentage calculation where m1 = mass of the sample and m2 = mass of the precipitate
What equipment and expertise are required for Sulphate content testing?
Equipment includes:
Coring equipment and a concrete saw for sample collection/preparation.
Mortar and pestle or ball mill to powder cores for chemical analysis.
- A precise electronic scale capable of measuring to at least 0.001 gram for weighing the samples.
250 ml and 400ml laboratory glass beakers and stirring rods.
Concentrated hydrochloric acid solution for decomposing the cement sample usually 37% HCl by mass.
Silver Nitrate solution for chloride ion disappearance test.
Barium Chloride Solution usually 12% BaCl2 by mass.
A heating source such as a Bunsen burner or electric hot plate.
Ashless filter paper.
A pH meter or litmus paper.
A muffle furnace capable of reaching (925 ± 25) °C for igniting the precipitate.
Significant experience and expertise in chemical laboratory testing is required to accurately carry out sulphate content testing and remain safe while using boiling liquids and strong acids. A strong understanding of the typical composition of concrete and the allowable ranges for sulphates is also required to successfully interpret the data and allow assessment of what results mean for structures in the real world.
What are the advantages of Sulphate content testing?
Sulphate content testing is a definitive measure of sulphate attack.
Accurate and relatively cheap test method that can be completed within one day.
Can identify the early stages of sulphate attack and allow preventative measures to be implemented before damage has occurred.
Repeating testing across the same element or structure can map sulphate attack and identify the high-risk areas most in need of protection.
The test can identify both internal and external sulphate attack and if repeated at different depths to create a sulphate profile map can differentiate between the two.
What are the disadvantages of Sulphate content testing?
The test method is complex requiring laboratory skills and equipment.
Conducting the test requires significant skill and there are many opportunities for human error to skew the results.
The test requires coring and repair of the structure.
The test requires the use of strong acids and boiling liquids which can pose significant health and safety risks if not properly controlled.
How accurate is Sulphate content testing?
According to BS EN 196-2:2013 correct procedures are followed the standard deviations for repeatability and reproducibility of this test are 0.07% and 0.08% respectively
What are the limitations of Sulphate content testing?
Assessing sulphate content in concrete structures faces several limitations.
First, certain sulphate sources, like sulphides in aggregates, might go undetected.
Sampling challenges arise in capturing a representative snapshot of overall sulphate content, especially in extensive or complex structures.
Surface-level tests may miss deeper damage, and sulphate levels' variability over time complicates the accuracy of single assessments for predicting long-term durability.
Moreover, these tests don't directly reveal structural damage, necessitating further examination.
Ancillary information
Maturity of test: > 10 years
Qualification & interpretation : Specialised lab
Service disruption: No
Preliminary works: No
Time consumption Medium (one day)
Cost Low
Access to element 1 face
References and further information









